Fossil fuelsFossil fuels, coal, oil and natural gas, are a non-renewable source of energy. Formed from plants and animals that lived up to 300 million years ago, fossil fuels are found in deposits beneath the earth. The fuels are burned to release the chemical energy that is stored within this resource. Energy is essential to moden society as we know it. Over 85% of our energy demands are met by the combustion of fossil fuels. These two pie charts show exactly how vital fossil fuels are to our society by showing how much of each energy resource is consumed.
Formation
Going back to the earlier days of Earth, the plants and animals that lived then eventually died and decomposed. The majority of these life forms were phytoplankton and zooplankton. When these ancient ocean dwellers died, they accumulated on the bottom of a seabed; this is how a good portion of our fossil fuel reserves began. The actual transformation process of these prehistoric creatures is not known, but scientists do know that the pressure, heat, and a great deal of time go into the making of fossil fuels.
Geologists are fairly certain that the beds of organic remains mixed with silt and mud to form layers. Over time, mineral sedimentation formed on top of the organisms, effectively entombing them in rock. As this occurred, pressure and temperature increased. These conditions, and possibly other unknown factors, caused organic material to break down into the simpler form of hydrocarbons: chains of carbon and hydrogen ranging from simple configuration to complex compounds. Another affect of extreme pressure is that the oil and gas which are various mixtures of hydrocarbons, migrate upwards to the surface. Exactly when in the conversion process and the nature of this migration is not known and is subject to conjecture.
Oil and gas are found in the ground, not freely drifting up through the earth. This is because the hydrocarbons come across rock formations that they are unable to penetrate. Complex rock structures that effectively trap gas and oil are formed by tectonic plate activity, the same forces that shift continents. The most common formation that accomplishes this is called an anticline, a dome or arched layer of rock that is impermeable by oil and gas. Underneath this barrier, a reservoir builds up. An oil reservoir is not some vast underground lake, but rather a seemingly solid layer of rock that is porous. Oil fields have been found everywhere on the planet except for the continent of Antarctica.
These fields always contain some gas, but this natural gas, methane, does not take nearly as long to form. Natural gas is also found in independent deposits within the ground as well as from others sources too. Methane is a common gas found in swamps and is also the byproduct of animals' digestive system. Incidentally, Methane is also a greenhouse gas.
Coal is formed in a similar to the other fossil fuels, though it goes through a different process, coalification. Coal is made of decomposed plant matter in conditions of high temperature and pressure, though it takes a relatively shorter amount of time to form. Coal is not a uniform substance either, it's composition varies from deposit to deposit. Factors that cause this deviation are the types of original plant matter, and the extent the plant matter decomposed. There are over 1200 distinguishable types of coal. Coal begins as peat, a mass of dead and decomposing plant matter. Peat itself has been used as fuel in the past, as an alternative to wood. Next, the peat becomes lignite, a brownish rock that contains recognizable plant matter and has a relatively low heating value. Lignite is the halfway point from peat to coal. The next phase is subbituminous. A shade of dull black, showing very little plant matter, this type of coal has a less than ideal heating value. Bituminous coal is jet black, very dense, and brittle. This type of coal has high heating value.
The main point of this is that all of these fossil fuels are made of hydrocarbons. It may come as a surprise that these two elements, hydrogen and carbon, can create many, many different compounds with unique characteristics. What makes hydrocarbons valuable to our society is the stored energy stored within them. This energy is contained in the atomic bonds. The original source of this energy is all the solar energy the prehistoric organisms trapped in their bodies eons ago. How do we make use of this bond energy then? We burn them.
Combustion, Drilling, and Refining
Combustion is the process of breaking atomic bonds to release energy in the form of light and heat. Fossil fuels have many hydrocarbons, each with numerous bonds. When they undergo combustion, they release a great deal of heat. This is the main reason why natural gas and heating oils are used extensively in the world today. However, energy in the form of heat is by nature very chaotic and disorganized. Simply burning fossil fuels is wonderful for keeping the winter chill at bay, but setting oil on fire in your washing machine won't get your clothes clean. Likewise, we can't put petroleum directly out of the ground into our cars and expect them to operate. To make use of the resource of fossil fuels, humans have developed drilling, refining, and methods to harness fossil fuel energy.
Early oil explorers relied heavily on intuition and guesswork to find the precious 'black gold.' These daring entrepreneurs were known as 'wildcatters.' A fabled technique used by the wildcatters is the 'old hat.' They would basically toss their hat up in the air and wherever it landed, they drilled. When the wildcatters got lucky, and struck oil, it would typically gush up the drill pipe, hence, a gusher. Because gushers are a safety hazard and environmental concern, oil companies today contain them. After discovering an oil field, it is the task of the oil company's engineers and technicians to get it out. Not all oil fields turn out to be gushers and even the ones that are eventually loose pressure, leaving a lot of untapped fossil fuel resource in the reservoir. Even with modern extraction techniques, 100% of the oil in any given field is still not yet recoverable.
One thing an oil company does to facilitate the extraction process is setting up what is known as a 'Christmas tree,' a system of valves and pipes that regulate oil flow and pressure. Another system used in much smaller reservoirs not worth the expense of manning with technicians is the setup of a beam pump These are also known as 'nodding donkeys;' they extract oil from small oil pools that do not contain much resource. In large oil fields, techniques such as water and gas injection are employed to maximize return of the investment. By pumping water and gas into the wells, the pressure increases allowing oil to flow upwards once more Large oil fields can be found under the sea floor as well. To exploit these fields, vast oil drilling stations, which are marvels of modern engineering, tap into these underwater deposits and bring them to the surface.
Although fossil fuels have been around long before humans even discovered fire, our prehistoric ancestors had no use for them. In the late 1800's, coal and gas were used as heat and light sources, steam locomotives as well. There were early automobiles too, but these vehicles were more of a novelty than a way of life. It wasn't until the 1940's did things change. Why the 1940's? The answer is that engineers and inventors had government support and extra incentive to develop fossil fuel technologies, war. World War II was the catalyst and not World War I because 'The War to End All Wars' was fought by men in trenches and mechanized warfare had only been developed late in the conflict. World War II had the German Blitzkrieg, or 'Lightning War.' This tactic utilized Shtuka dive bombers and Panzer tanks; German engineers enabled this, and was eventually countered by Allied technological advancements. From then on, usage and development of fossil fuels steadily rose.
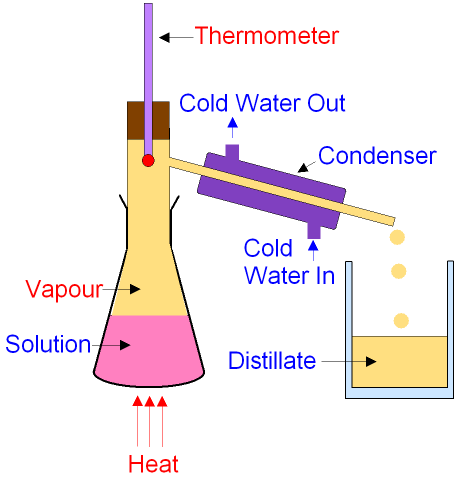
The primary refining technique used to separate hydrocarbons and provide the ingredients for modern fuels is called fractional distillation. Hydrocarbons of different size and configuration usually have differences in boiling points that are large enough to use as a method of separation. By vaporizing them, they tend to float upwards until the hydrocarbons condense, which is where they are collected. Hydrocarbons as simple as butane and alcohols with few carbons are sorted along with more complex ones such as aromatics with 9 carbons. The fuels we commonly use today are a mixture of these hydrocarbons distilled from the petroleum extracted from the earth.
Fuel types and Engines
Gasoline is a highly specialized fuel that contains hydrocarbons ranging from butane to C10. It is designed for the Otto-cycle engine, also known as spark ignition or 4-stroke engine. This engine as well as others will be described in more detail later on. Some characteristics of gasoline enable the following:
1. Quick start at low temperatures
2. Fast acceleration
3. Low occurrence of stalling
4. Relatively quiet and low tendency to knock
5. Good combustion efficiencyThe next classification of fuels is the distillate fuels. They are kerosene, turbo-jet fuel, diesel, and heating oil. Kerosene was the first petroleum fuel oil to be widely used; this was before electric lights and after the days of animal and vegetable oil. Kerosene has become less popular and is no longer produced in the quantities it once was. Countries with limited access to electricity and outdoors enthusiasts still have a use for this fuel.
Turbo-jet fuel was first developed in WWII for use in airplane engines. Because of constraints on petroleum products, namely gasoline for tanks and other ground vehicles, this fuel was designed to make use of compounds not vital to gasoline production whenever possible. The result was a highly volatile fuel that led to many accidents in handling. Modern aviation fuel is still more volatile than gasoline, though it has become much safer than it previously was.
Diesel fuel and domestic heating oil are similar in composition. Domestic heating oils are not widely used in the US, though they still have limited application in underdeveloped countries. Diesel fuels are used frequently in the world today; transport vehicles such as trains, boats, trucks, and busses use diesel fuel.
Fuel oils are mainly residuals from the fractional distillation process. They are more or less the leftovers from production of other fuels. They have been and are still used in power generation plants. Because of the low quality and high pollution content fuel oils are being used less often.
Of the fuels previously listed, gasoline, turbo-jet fuel, and diesel fuel were designed for usage in engines. A fairly good, simple definition of an engine is a device that converts chemical or heat energy into mechanical energy. Engines convert fossil fuel energy into a form that we can more readily use.
The majority of engines in the world today are internal combustion engines. This type of engine
Friday, November 23, 2007
Subscribe to:
Post Comments (Atom)











No comments:
Post a Comment